 Quality Control system in Greenbo
Quality Control system in Greenbo
The fundamental principle:Technology pilot, high-quality, safety, pollution prevention, environmental protection, regulation compliance, continuous improvement.
In Greenbo, the quality management system aims to meet customer needs and increase customer satisfaction. The QA, GMP, QC and QRM departments are the integral parts of quality management system. The top leaders and different departments are included in the quality management system.
An independent quality system has been set up to realize the function of Greenbo’s quality management, which includes regulatory affairs and applied research and development. The regulatory compliance and the approval of product market access have been sped up by the complementary advantages of quality management and regulatory affairs.
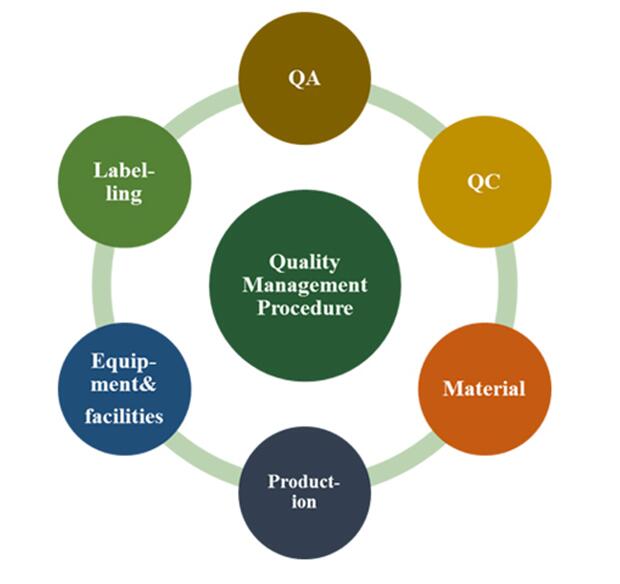
Quality principle: 1. Science/Risk-based quality management 2. Scientific concepts throughout the quality management 3. Quality management facilitates the continual improvement.
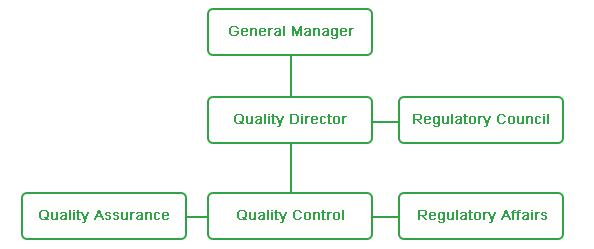
QC Department
Everything at Greenbo proves that “Quality is our life”. Quality is strictly controlled by corresponding standard in each procedure from selection of material to product delivery. Professional test equipments like HPLC/GC/UC are used and full-time quality control. Inspection data like ingredient, moisture and dust content and heavy metal residual, and solvents are documented completely and in detail.
QA Department
Safety and effective active ingredients in plants are reserved from the collection of starting material, griding and extraction to finished products. Proven quality management system ensures strict compliance with GMP requirements at each procedure. A detailed and complete record is maintained and documented appropriately for each batch of products to ensure steady quality.
Greenbo Record Management Office
The company's quality management system meets the requirements of different management departments, such as the Ministry of Agriculture of China, US FDA, Australian APVMA, European Authorities and Supplier Audit etc. In the past 6 years, the company has passed different official inspections and audits for about 20 times.


 Quality Control system in Greenbo
Quality Control system in Greenbo


